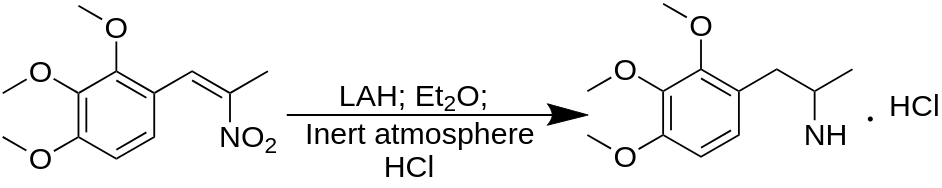
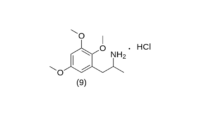
TMA-3 synthesis
This is classical synthesis method of TMA-3 was crated with help of experts from BB forum.
Method
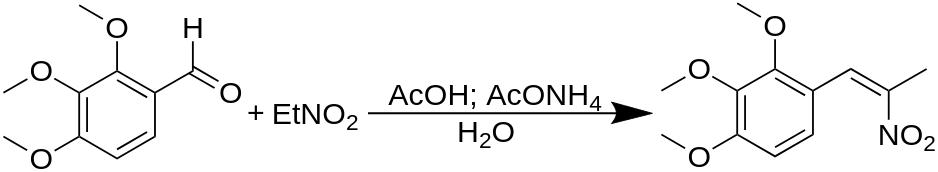
To a solution of 12.4 g 2,3,4-trimethoxybenzaldehyde in 45 mL glacial acetic acid, there was added 7 mL nitroethane and 4.1 g anhydrous ammonium acetate, and all was held at reflux temperature for 1.5 h. To the cooled and well stirred reaction mixture, H2O was added slowly, dropping out an oily crystalline solid mass. This was separated by filtration, and ground under a quantity of 50% aqueous acetic acid, and re-filtered. The 6.5 g of crude product was recrystallized from boiling MeOH to give, after air drying to constant weight, 5.0 g of 2-nitro-1-(2,3,4-trimethoxyphenyl)propene, with a m.p. of 56-57 °C.
To a gently refluxing suspension of 3.0 g LAH in 300 mL anhydrous Et2O under a He (or nitrogen N2) atmosphere, there was added 3.65 g 2-nitro-1-(2,3,4-trimethoxyphenyl)propene by allowing the condensing Et2O drip into a shunted Soxhlet thimble containing the nitrostyrene and effectively adding a warm saturated solu-tion of it dropwise. Refluxing was maintained for 5 h following the completion of the addition of the nitrostyrene. The milky reaction mixture was cooled and the excess hydride destroyed by the addition of 200 mL 10% H2SO4. When the aqueous and Et2O layers were finally clear, they were separated, and 75 g of potassium sodium tartrate was dissolved in the aqueous fraction. NaOH (25%) was then added until the pH was >9, and this was then extracted with 3×75 mL CH2Cl2. Evaporation of the solvent under vacuum produced 2.5 g of a nearly colorless clear oil that was dissolved in 300 mL anhydrous Et2O which was saturated with anhydrous HCl gas. The product, 2,3,4-trimethoxyamphetamine hydrochloride (TMA-3) separated as a fine white solid. This was removed by filtration, Et2O washed, and air dried to constant weight. The yield was 1.65 g of a product which, after recrystallization from IPA, had a m.p. of 148-149 °C.