TMA (Trimethoxyamphetamine) assessment protocol
Introduction
You bought TMA but you are not sure what exactly TMA do you have and is it real TMA? You want to figure out what did you bought. Then you open this article and use it as a guide for experimenting. There are list of manipulations with TMA and useful information for home tests.
There are six TMAs:
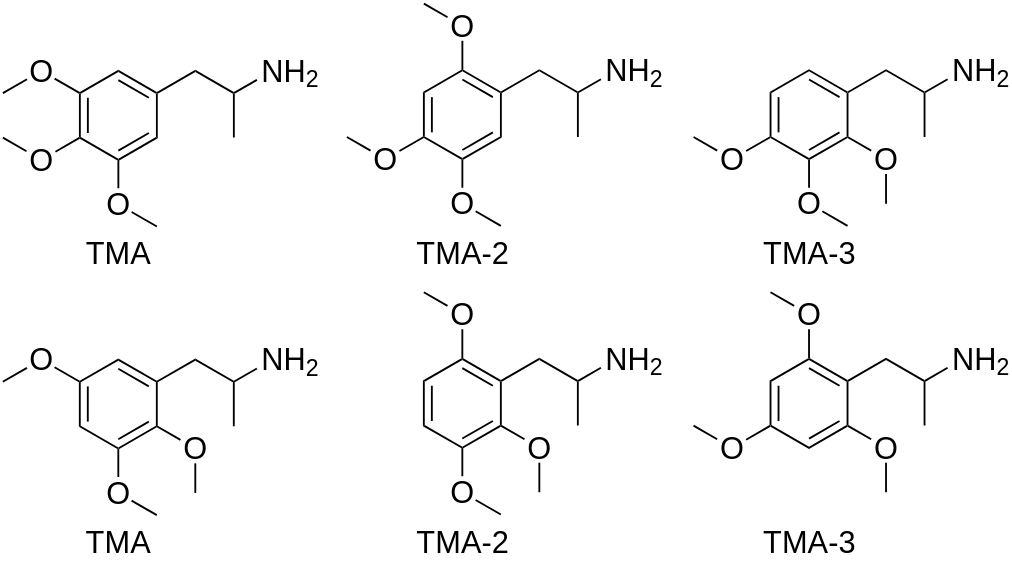
Possible side products and adulterant substances
In a list of possible admixtures are mescaline, 2C-x and DOx substances, LSD by reason of similar psychedelic effects.

Algorithm of procedures:
1. Firstly, you have to provide visual revision of your TMA. If your product has different color from white, It, probably, has some organic or inorganic syntheses side products, which can change color. The color of TMA depends on reactions and their quality, which was carried out. More colored means a dirtier product.
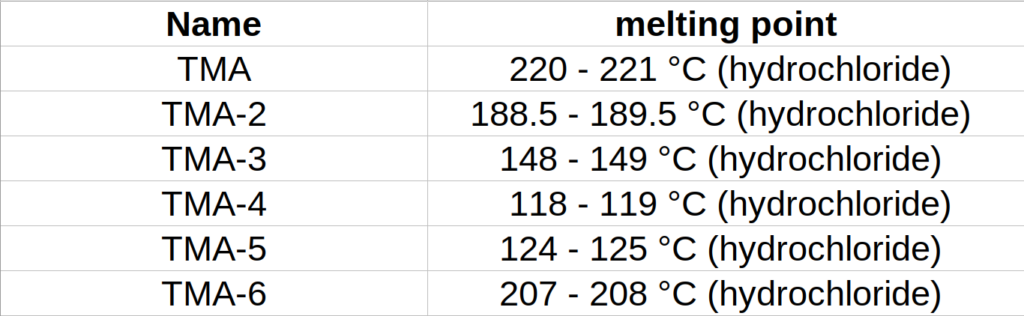
2. Secondary, measure melting point of your product to determine which TMA do you have. Melting point determination manual can be learned from mentioned topic.
3. Thirdly, confirm the compliance of the product with the declared amphetamine by LF tests (drug testing kits). You will receive clear result about your substance and admixtured narcotic substance (if it takes place to be) and these tests help to chpose next step. You have to obtain only amphetamine as a result.
4. Next, if you are not sure in your melting point result and you are still doubt in your TMA or LF tests reveal admixtured narcotic substance, lead experiments with test reagents. Use «Drugs testing reagents». These methods help to determine kind of admixtures. There are manuals, which are described checking procedures and meaning, where you can find methods of reagent synthesis. According to data from testing reagent experiments, you may compare and approve result by TLC.

5. As a last confirmatory experiment I found following method, which allows to determine methoxy groups in your product. It is quite complicated method but may be interesting for some people.
Qualitative test for methoxy and other alkoxy groups
Preparatioxof porousplugs
Cut double-thickness cheese cloth (gauze) from a roll 18 inches (45 cm) wide into strips 2 inches (5 cm) wide and 18 inches long. Fold each strip once, to get a strip 2 by 9 inches. Place the strips on a sheet of glass. Thoroughly moisten each strip by pipetting 5 ml. of the impregnating solution onto it, taking care that the strips do not curl. Dry the strips on the glass, either by standing overnight or by leaving it in a warm place for 2 or 3 hours.
Impregnatingsolution
Dissolve 1 gram of lead acetate in about 10 ml. of water and pour it into 60 mL of 1 N sodium hydroxide in a 100- mL cylinder. The heavy precipitate which appears soon redissolves. Add a solution of 5.0 grams of sodium thiosulfate in about 10 mL of water, and 1 mL of glycerol. Dilute the mixture to 100 mL with water. The glycerol prevents any of the dried salt mixture from flying off as dust when the strips are rolled before insertion in the test tubes. Place about 0.1 g of the substance to be tested (preferably well powdered if a solid), in a 16 by 150 mm. test tube. Carefully pipet 1 ml. of glacial acetic acid and 1 mL of hydriodic acid (57 %, sp. gr. 1.7) onto it. It is desirable, but not essential, to add a small (1 to 2 mm) granule of unglazed porcelain to promote ebullition. Roll one of the im pregnated gauze strips rather loosely into a cylindrical shape and insert it in the mouth of the test tube with a rotary motion. When most of the plug has been inserted into the tube, twist it in the opposite direction to expand it in the bore to a fairly tight fit, There must be no obvious channels by which vapors can bypass the plug. Push the plug down until its top is about 1.5 inches (4 cm) below the mouth of the test tube. Put a small piece of nonabsorbent cotton (cotton batting) over the plug and press it down into a disk about 2 to 3 mm thick. Give a strip of filter paper about 2 cm wide and 10 cm long a slight longitudinal fold, moisten about a third of its length with a saturated solution of mercuric nitrate in 2 % by volume nitric acid, and rest the paper upon the cotton wad. Place the test tube in a glycerol bath about 4 to 5 em. deep, maintained at 120 to 130 °С. The bath may be heated initially above 130 °С, if several tubes are to be immersed in it. Condensed vapors will gradually work up the tube walls into the impregnated plug, which will show a gray discoloration.
With a positive test, a yellow color will spread upward from the bottom edge of the test paper, while changing to a bright orange or vermilion. Less frequently, the orange or vermilion color will appear rather suddenly over all the moist portion of the test paper. The vermilion may appear as a solid band of color, or as spots against a background of yellow or yelloworange. A permanent yellow color indicates a negative or a doubtful positive test. The faint yellowish color obtained when the mercuric nitrate solution dries on the test paper is without significance.
The test should be continued until a distinct positive reaction is evident, or until 10 minutes have elapsed. I n certain cases, particularly in tests which have been heated for 10 minutes with negative results, a slight gray discoloration may appear on the test paper, probably owing to the action of volatile substances which slightly reduce the mercuric nitrate with the production of finely divided mercury. This discoloration usually fades in an hour or so. Permanent dark gray discolorations due t o sulfur compounds are discussed under “Results”.
Method limits of sensitivity
The test was applied to 5-, 10-, and 25-mg. portions of vanillin and codeine (alkaloid). The colors were fairly strong with the 25 mg portions. With vanillin the test was still positive, although weak, with 5 mg. With codeine, the test was still positive (moderate orange color) with 10 mg but 5 mg gave only a lemon-yellow color. Accordingly, the limits of sensitivity are about 5 mg for vanillin and 10 mg for codeine.


