TMA-2 synthesis
This is classical synthesis method of TMA-2 was made with experts from BB forum.
Method
To a solution of 50 g 2,4,5-Trimethoxybenzaldehyde in 175 mL nitroethane there was added 10 g anhydrous ammonium acetate and the mixture was heated on the steam bath for 2 h. The excess nitroethane was removed under vacuum, and the deep orange oily residue was drained out into a beaker, and the flask washed with 3×60 mL boiling MeOH. On stirring the combined decantation and washings, there was a spontaneous formation of crystals. After cooling, these were removed by filtration, washed sparing with MeOH, and air dried to constant weight to yield 35.1 g of 2-nitro-1-(2,4,5-trimethoxyphenyl)propene as yellow crystals with a m.p. of 98–99 °C. Recrystallization from MeOH increased the m.p. to 101–102 °C.
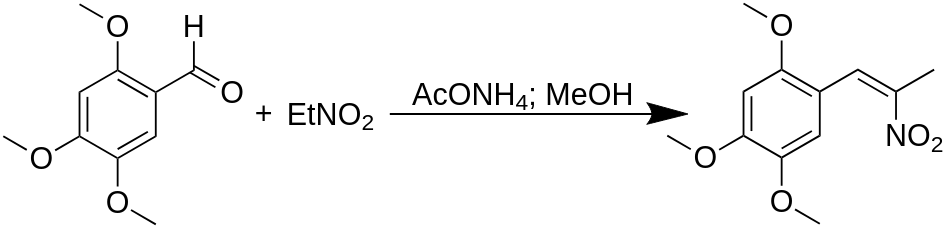
A suspension of 31.6 g powdered LAH in 1 L anhydrous THF containing a little anhydrous Et2O was brought to a gentle reflux, and then there was added a solution of 40.0 g of 2-nitro-1-(2,4,5-trimethoxyphenyl)propene in 200 mL anhydrous THF over the course of 4 h. The mixture was held at reflux temperature for 24 h, cooled to 0 °C with external ice, and the excess hydride destroyed by the addition, in sequence, of 32 mL H2O (which had been diluted with a little THF), 32 mL 15% NaOH, and finally with 96 mL H2O. The white inorganic solids were removed by filtration, and the filter cake was washed with THF. The combined filtrate and washings were stripped of solvent under vacuum to give 48 g of an impure amber oil. This was dissolved in 180 mL IPA, neutralized with 30 mL concentrated HCl, and the mixture diluted with 1500 mL anhydrous Et2O. After a short induction period, an oily precipitate separated, which on stirring changed into a loose crystalline phase. This was removed by filtration, washed with Et2O, and air dried to yield 29.0 g of 2,4,5-trimethoxyamphetamine hydrochloride (TMA-2) as fine white crystals with a m.p. of 188.5–189.5 °C. A 4.0 g sample of the free base was dissolved in 15 mL pyridine, treated with 2.5 mL acetic anhydride, heated on the steam bath for 20 min, added to 400 mL H2O, acidified with HCl, and extracted with 3×75 mL CH2Cl2. After washing with H2O the pooled extracts were stripped of solvent under vacuum to give 4.5 g of flakey, off-white solids which, on recrystallization from MeOH, were white, weighed 2.3 g, and had a mp of 132–133 °C. Recrystallization from this acetamide from MEK did not improve its quality.